Medicine has taken a new step forward with the approval of CASGEVY (exagamglogene autotemcel) in Europe, the first gene therapy based on CRISPR-Cas9 gene-editing technology. This breakthrough marks a significant milestone in the treatment of genetic diseases such as beta-thalassemia and sickle cell anemia, hereditary disorders that severely affect hemoglobin production and have had limited therapeutic options until now.
What is CASGEVY?
CASGEVY (exagamglogene autotemcel) is a gene therapy approved in Europe for the treatment of sickle cell anemia, a genetic disease that affects hemoglobin production in the body. This condition causes severe symptoms and long-term health complications. CASGEVY offers an innovative option beyond conventional treatments like blood transfusions and chelation therapies.
The therapy works by extracting hematopoietic stem cells from the patient, which are then genetically modified in the lab to correct the mutation causing the disease. These edited cells are reintroduced into the patient, with the goal of integrating and starting to produce healthy blood cells, offering a potentially long-term and curative treatment.
How does CRISPR-Cas9 technology work?
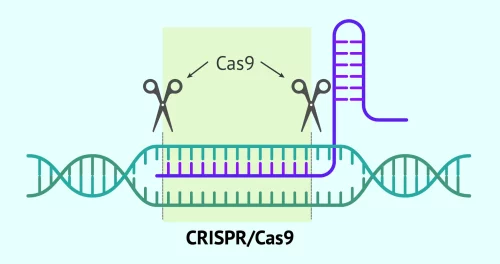
CRISPR-Cas9 technology has revolutionized biotechnology with its ability to precisely and efficiently edit the genome. CRISPR, which stands for "Clustered Regularly Interspaced Short Palindromic Repeats," is a tool that allows scientists to cut DNA at specific locations and make changes to genetic sequences. Cas9, an enzyme, acts like molecular scissors that cut DNA, allowing researchers to remove, add, or alter sections of the genome.
This system is critical for gene therapies where precision is fundamental to ensure that genetic changes do not cause unwanted effects. In the context of gene therapy, CRISPR-Cas9 offers a pathway to treat genetic diseases, although not all therapies, like CASGEVY, necessarily use this technology. The ability to directly modify DNA in living cells opens possibilities for treating a wide variety of genetic disorders previously considered untreatable, promising a new era of personalized medicine and treatments tailored to each patient's genetic profile.
How biotechnology is driving gene therapy innovation
Gene therapy has been on researchers' radar for decades, but with the advent of CRISPR-Cas9, the field has experienced unprecedented acceleration. This technique allows not only the treatment of symptoms but also targets the genetic root of the problem, directly correcting the mutation that causes the disease. CASGEVY represents the future of personalized medicine, where treatments are specifically designed for each individual.
Here is where the technology and equipment used in the biotechnology industry play a crucial role. In stem cell manipulation, advanced bioreactors and filtration systems, such as those provided by TECNIC, are essential. Bioreactors allow cell expansion under controlled conditions, ensuring the purity and viability of the edited cells, while Tangential Flow Filtration (TFF) ensures the proper separation and purification of biological products like the modified cells.

Approval in Europe and the United States
Both the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) have approved CASGEVY for the treatment of beta-thalassemia and sickle cell anemia. The FDA's approval in the United States underscores the therapy's efficacy and reinforces the idea that gene editing with CRISPR-Cas9 is a viable and safe approach to treating genetic diseases. With both regions giving their approval, CASGEVY is now available to patients in Europe and the U.S., representing a significant step toward the globalization of CRISPR-based gene therapy.
Conclusion
The approval of CASGEVY in Europe marks a key moment in the history of medicine and biotechnology, offering new hope to thousands of patients. CRISPR-Cas9 technology not only revolutionizes the treatment of genetic diseases but also opens the door to future therapies that could change the course of previously incurable conditions.
Biotechnology is advancing rapidly, and companies like TECNIC play a crucial role in supporting and facilitating the development of these innovations by providing advanced equipment, from bioreactors to filtration systems. With each new advance, we are getting closer to an era where genetic diseases become a thing of the past.
Frequently Asked Questions (FAQ)
CASGEVY is the first gene therapy approved in Europe based on CRISPR-Cas9 technology, used to treat sickle cell disease and beta-thalassaemia.
CRISPR-Cas9 is a gene-editing tool that allows scientists to cut and modify DNA at specific sites, correcting genetic defects.
Cas9 is an enzyme that acts like a ‘molecular scissors’, cutting the DNA at the exact place where the gene correction is needed.
CRISPR-Cas9 offers unprecedented precision in gene editing, allowing targeted DNA changes to be made more efficiently and with less risk of error.
Yes, both the FDA in the US and the EMA in Europe have approved CASGEVY, allowing its use in both regions.
Sources:
- European Medicines Agency. (2023). First gene editing therapy to treat beta-thalassemia and severe sickle cell disease
- Science Media Centre. (2023). Reaction to EMA gives green light to EU’s first CRISPR gene-editing drug
- CRISPR Therapeutics. (2023). European Commission approves first CRISPR-Cas9 gene-edited therapy