Breast cancer is one of the most prevalent neoplasms worldwide and remains a leading cause of mortality among women. Despite advancements in targeted therapies, treating metastatic HR-positive, HER2-negative breast cancer remains a clinical challenge. In this context, the approval of Datroway (datopotamab deruxtecan-dlnk) by the FDA in January 2025 marks a milestone in the fight against this disease, providing an innovative alternative for patients previously treated with endocrine therapy and chemotherapy.
Mechanism of action of datroway
Datroway is an antibody-drug conjugate (ADC) targeting the TROP2 protein, a surface receptor overexpressed in various epithelial tumors. Its design allows for highly selective action on tumor cells while minimizing toxicity in healthy tissues.
The drug combines three key elements:
Anti-TROP2 monoclonal antibody: Facilitates the selective internalization of the drug into tumor cells by binding to the TROP2 protein on the cell membrane.
Topoisomerase I inhibitor (DXd): A cytotoxic molecule that disrupts DNA replication, inducing apoptosis in tumor cells.
Stable yet cleavable linker: Ensures the drug is specifically released inside malignant cells, optimizing efficacy and reducing systemic toxicity.
This approach improves the efficacy-to-toxicity ratio compared to conventional chemotherapy and represents a significant advance in developing targeted therapies for metastatic breast cancer. Furthermore, the molecular engineering applied in Datroway enables the drug to bypass common resistance mechanisms, increasing its effectiveness even in patients who have developed tolerance to other treatment lines. Additionally, the drug’s pharmacokinetics and pharmacodynamics have been optimized to ensure a prolonged half-life, maximizing its impact while minimizing dosing frequency.
Recent studies have highlighted that TROP2 expression is not exclusive to breast cancer but is present in multiple epithelial malignancies, making Datroway a potential candidate for broader oncological applications. Researchers are actively exploring how variations in TROP2 expression correlate with treatment response, paving the way for more refined, biomarker-driven treatment strategies.
Clinical evidence: TROPION-Breast01 study
The approval of Datroway was based on findings from the phase III TROPION-Breast01 clinical trial, which evaluated the efficacy and safety of the drug in patients with metastatic HR-positive, HER2-negative breast cancer previously treated with endocrine therapy and at least one line of chemotherapy.
Key study results include:
Progression-free survival (PFS): 6.9 months in the Datroway-treated group vs. 4.9 months in the standard chemotherapy group.
37% reduction in the risk of disease progression or death compared to conventional chemotherapy.
Objective response rate (ORR): 36.4% with Datroway vs. 22.9% with chemotherapy.
Manageable safety profile, with known and mostly low-grade adverse effects. Among them, interstitial lung disease (4.2%) and mild keratitis were the most reported events.
These results confirm that Datroway is not only a viable option but also a significant improvement in the quality of life for patients with advanced breast cancer, offering a more tolerable and effective alternative than conventional treatments. Notably, patients treated with Datroway experienced a lower incidence of severe adverse events, suggesting better long-term tolerability. Additional long-term follow-up studies are currently assessing the potential benefits of extended Datroway treatment, including its impact on overall survival and quality of life metrics.
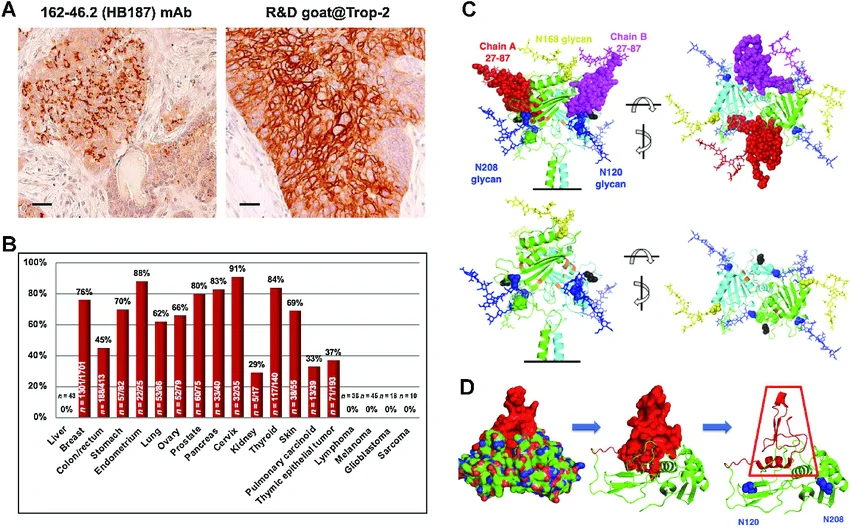
Implications for breast cancer therapeutics
Metastatic HR-positive, HER2-negative breast cancer is the most common subtype, accounting for approximately 70% of cases. While endocrine therapy remains the first-line treatment, eventual resistance to these therapies limits their effectiveness, necessitating chemotherapy, which has reduced efficacy and significant toxicity.
Datroway provides an alternative with a highly targeted mechanism of action and lower toxicity compared to traditional chemotherapy. Its introduction reinforces the growing relevance of ADCs in modern oncology, offering a more precise approach to treating metastatic solid tumors. The increasing use of ADCs suggests a paradigm shift in how oncologists approach treatment-resistant tumors, where combination strategies with immunotherapies or additional targeted agents are being actively explored.
Additionally, ADCs are gaining prominence in other oncology areas. Datroway is currently being evaluated in clinical trials for non-small cell lung cancer and other solid tumors, suggesting a promising future for this type of targeted therapy. Preclinical studies have indicated that Datroway's optimized design allows its use in combination with immune checkpoint inhibitors, which could further enhance its therapeutic efficacy and open new avenues for combination treatments. There is also growing interest in utilizing Datroway in earlier treatment settings, potentially reducing the need for aggressive chemotherapy regimens in patients with earlier-stage disease.
Datroway's impact extends beyond clinical outcomes to the perspective of personalized treatment. Through biomarker research, it is possible to predict which patients will respond best to this drug, enabling better treatment selection and avoiding the unnecessary administration of therapies with a lower likelihood of success. Molecular profiling technologies are becoming integral to clinical decision-making, allowing oncologists to tailor treatments based on an individual patient’s tumor characteristics, increasing therapeutic success rates while minimizing adverse effects.
Conclusion
The approval of Datroway represents a significant advancement in treating metastatic HR-positive, HER2-negative breast cancer. Its design as an antibody-drug conjugate allows for specific action on tumor cells, minimizing the adverse effects associated with conventional chemotherapy.
The implementation of advanced bioprocessing technologies is essential for manufacturing ADCs like Datroway. Companies like TECNIC play a crucial role in optimizing these processes, enabling the efficient and scalable production of innovative therapies. The combination of advancements in biotechnology with more specific therapeutic approaches paves the way for a new era in cancer treatment. The expanded use of Datroway and other ADCs in oncology highlights the positive impact of these therapies on patients' quality of life, emphasizing the importance of continued research and development in this field.
As ADC technology continues to evolve, the role of next-generation bioprocessing techniques, enhanced linker stability, and even new payload delivery mechanisms will further refine the effectiveness of treatments like Datroway. The success of this drug exemplifies how precision medicine, advanced biotechnology, and rigorous clinical research are revolutionizing oncology, bringing forth therapies that offer improved survival while minimizing the burdens associated with traditional cancer treatments.

Frequently Asked Questions (FAQ)
Datroway (datopotamab deruxtecan-dlnk) is an antibody-drug conjugate (ADC) targeting TROP2, a protein overexpressed in tumors. It delivers a cytotoxic agent directly to cancer cells, reducing damage to healthy tissues.
It selectively targets cancer cells, minimizing side effects and improving effectiveness compared to traditional chemotherapy.
Patients with metastatic HR-positive, HER2-negative breast cancer who have received endocrine therapy and at least one chemotherapy line.
Mild fatigue, nausea, keratitis, and interstitial lung disease (4.2%). Most are low-grade and treatable.
It reinforces targeted therapies, offering better precision and fewer side effects than chemotherapy.
Source of information
U.S. Food and Drug Administration. (2025). FDA approves datopotamab deruxtecan-dlnk for unresectable or metastatic HR-positive, HER2-negative breast cancer. FDA.
Wen, Y., Ouyang, D., Zou, Q., Chen, Q., Luo, N., He, H., Anwar, M., & Yi, W. (2022). A literature review of the promising future of TROP2: A potential drug therapy target. Annals of Translational Medicine, 10(24), 1403.
Guerra, E., Trerotola, M., Relli, V., Lattanzio, R., et al. (2023). 3D-informed targeting of the Trop-2 signal-activation site drives selective cancer vulnerability. Molecular Cancer Therapeutics, 22(6).