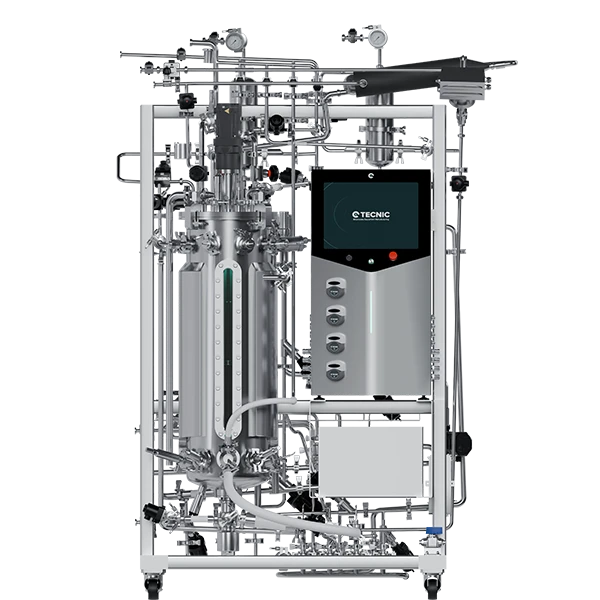
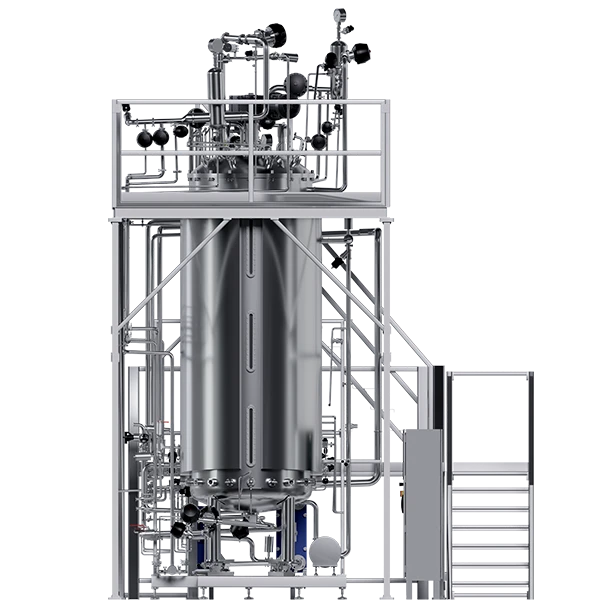
In the field of biotechnology, the terms fermenter and bioreactor are frequently used but often confused. Although both devices are used for the cultivation of micro-organisms and cells, they have important differences in design, applications, and technical specifications. Understanding the difference between a fermenter and a bioreactor is crucial for selecting the right equipment for the specific needs of the process.
Fermenter
A fermenter is a piece of equipment specifically designed to carry out fermentation processes, where microorganisms such as bacteria, yeasts or fungi transform organic substances into products such as alcohol, organic acids and gases. Fermenters are common in the food and beverage industry, but also have applications in the chemical and pharmaceutical industry.
Key features of a fermenter
Fermenters are used especially in the production of fermented foods and beverages such as beer, wine, yoghurt and fermented soy products. In the pharmaceutical industry, they are used to produce antibiotics and other biotechnological products by microbial fermentation.
- Specific application: Designed primarily for fermentation processes.
- Control of conditions: Allows precise control of factors such as temperature, pH and oxygen, essential for fermentation.
- Materials of construction: Generally made of stainless steel to prevent contamination and facilitate cleaning.
- Process types: Handles both aerobic (with oxygen) and anaerobic (without oxygen) processes.
Bioreactor
A bioreactor ⇀ is a more versatile piece of equipment used for a variety of bioprocesses, not just fermentation. It is used in the production of biotechnological products such as proteins, vaccines, monoclonal antibodies and stem cells. Bioreactors can grow animal and plant cells, as well as microorganisms.
Key features of a bioreactor
Bioreactors have different applications in the pharmaceutical ⇀ industry for the production of drugs, such as monoclonal antibodies and vaccines. They are also used in the production of biofuels, bioplastics, and in stem cell and gene therapy research.
- Versatility: Used for a wide range of processes, not just fermentation.
- Scalability: It is very varied, ranging from small volumes in laboratories to large volumes for industrial production.
- Advanced control: Includes precise monitoring and control systems to optimise culture conditions, such as dissolved oxygen, CO2, pH and temperature sensors.
- Diverse applications: Used in the pharmaceutical industry, biofuel production, stem cell research, and more.
Main differences between a fermenter and a bioreactor
- Application: The fermenter is more focused on specific fermentation processes, while the bioreactor is more versatile and used in a variety of biological processes.
- Design and construction: Although both can be made of similar materials, bioreactors tend to have more advanced and complex control systems.
- Versatility: Bioreactors offer greater flexibility in terms of the types of organisms and processes they can support.
- Process control: Bioreactors are equipped with advanced control and monitoring technologies, allowing for accurate, real-time management of culture conditions. Fermenters tend to be less complex in comparison.
- Scalability: Both can be scaled up for industrial production, but bioreactors have an advantage in the variety of scales and process types they can handle, from research to commercial production.

Frequently Asked Questions (FAQ)
Yes, bioreactors are very versatile and can be used for fermentation processes in addition to other biological processes.
You should consider the type of biological process, the scale of production, the level of control required and the versatility needed for future projects.
Generally, bioreactors can be more expensive due to their versatility and the advanced control and monitoring systems they include.
The main advantage of a bioreactor is its versatility and ability to handle a variety of biological processes with strictly controlled culture conditions.
Yes, both fermenters and bioreactors can be scaled up from small volumes for research to large volumes for industrial production.