Growth factors occupy a central position in the field of bioreactor systems, as they help stimulate cell growth, proliferation and differentiation to substantially increase the production of desired cells or tissues. A wide range of approaches have emerged in the bioreactor technology landscape, each offering innovative methodologies for the seamless integration of growth factors into these systems:
What are growth factors?
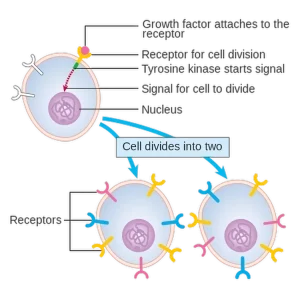
Growth factors are proteins that play a key role in stimulating cell growth, proliferation and differentiation. They act by binding to specific receptors on the cell surface, triggering processes that are essential for the development and repair of tissues in the body. These proteins are key to maintaining normal cell function and facilitating tissue recovery and regeneration in response to damage or disease.
In biotechnology, growth factors are crucial for optimising cell cultures in bioreactors, where they are used to control and maximise the production of specific cells or biological products. Their precise application enables replication of ideal conditions that promote efficient and uniform cell growth, which is essential for the large-scale production of advanced therapies and drugs. The integration of growth factors into bioreactor systems underlines their importance in the modern biotechnology and biopharmaceutical industry.
In addition, growth factors play a crucial role in regenerative medicine, a branch that focuses on healing or replacing damaged tissues and organs through the use of cell therapies and biomaterials. They are fundamental components in tissue engineering, where their precise application can direct the formation of tissue into three-dimensional structures. This opens up possibilities for treating a wide range of chronic diseases and injuries, from skin regeneration to organ repair. The ability to manipulate and apply these growth factors in clinical settings is transforming expectations for recovery in patients with conditions previously considered untreatable.
Strategies for integrating growth factors into bioreactors
- Direct Addition: Simply introduce growth factors into the bioreactor’s culture medium.
- Encapsulation: Encapsulate growth factors within biocompatible materials, such as hydrogels or microspheres, before introducing them into the bioreactor.
- Genetic Engineering: Genetically engineer cells to produce and release growth factors directly into the culture medium, ensuring continuous production throughout the culture period.
- Surface Immobilization: Attach growth factors to scaffold surfaces or bioreactor components.
The choice of method for incorporating growth factors into bioreactor systems depends on factors like the specific application, desired release kinetics, and compatibility with the cultured cells or tissues. Optimizing the delivery method is essential to ensure the efficient and effective utilization of growth factors in promoting cell growth and tissue development within the bioreactor system.
TECNIC’s Laboratory Bioreactors
Bioreactors are integral to bioprocesses, especially in the biopharmaceutical industry, where valuable materials are derived from microorganisms. The design of these systems is critical to achieving desired outcomes, as it is within bioreactors that microorganisms reproduce, grow, work, and fulfill their role. This underscores the significance of comprehensive research in designing equipment that provides optimal conditions for microorganisms.
At TECNIC, we offer solutions that empower you, starting from your laboratory bench up to full-scale production. You can start work with volumes ranging from 1 to 5 liters, automating the entire production process, thereby streamlining your work. Our objective is to support researchers and biotechnology professionals by equipping them with the tools necessary to achieve optimal results efficiently and under precisely controlled conditions.
Quality management is paramount in the industry, especially when working with growth factor products in immunotherapy research. The quality of recombinant proteins produced in vitro can vary due to a range of factors, from raw material quality to process inconsistencies. TECNIC equipment is manufactured under GMP compliance, ensuring high safety, purity, stability, and efficacy to promote therapeutic drug research and expedite global regulatory approval of biological products. We offer bioreactors in various scales, manufacture with the specific need to each process.