In the fast-paced world of biotechnology, achieving high efficiency and productivity in bioprocesses is crucial. Perfusion bioreactor is a new technique that is better than traditional batch and fed-batch methods in many ways.
By continuously exchanging the culture medium, perfusion systems keep cells in their optimal growth conditions. This not only boosts cell productivity but also improves the overall quality of the final product. Imagine being able to produce more with better consistency – that's the promise of perfusion technology.
Curious about how this works and what makes it so effective?
In this article, we’ll explore the basics of perfusion, its advantages, and the different types of perfusion systems. Whether you are experienced or new to bioprocessing, this guide will give you helpful tips to improve your processes.
What is perfusion?
Perfusion in cell culture is a method that maintains cells within a bioreactor while continuously exchanging the culture medium.
This process ensures that it replenishes nutrients and carbon sources with fresh medium, while removing cellular waste and depleted medium.
The exchange of medium is typically measured in vessel volumes per day (VVD). For example, perfusing 6 liters of medium daily into a system with a 3-liter working volume equals 2 VVD. This continuous refreshment of the medium helps sustain optimal cell growth and productivity.
Cell retention methods
In suspension culture, there are two primary methods for retaining cells during perfusion: filtration and settling. Each method has its unique advantages and challenges.
Filtration
Filtration methods, such as tangential flow filtration (TFF), circulate the medium from the bioreactor through porous hollow fibers.
These fibers are designed to allow the medium to pass through while retaining the larger cells within the reactor.
The spent medium (permeate) flows across the membrane and is removed, while the cells cycle back into the reactor vessel.
Filtration methods provide fully clarified permeate that can be directly linked to downstream processing. This makes them ideal for processes where maintaining high cell density and product purity is essential.
Sedimention
Sedimentation techniques use gentle flows to help cells sink and collect. The settled cells are then returned to the bioreactor, while the remaining liquid is removed.
Settling methods are generally more cost-effective and have a lower risk of system fouling compared to filtration methods. However, they are less efficient at retaining cells, often resulting in some cell loss (referred to as "bleed") in the spent medium. Despite this drawback, settling methods can be advantageous for processes with lower cell density requirements and where cost reduction is a priority.
Types of perfusion
N-1 Perfusion
N-1 perfusion, also known as intensified seed train perfusion, focuses on achieving high cell densities while cells are in their logarithmic growth phase.
Usually, this procedure takes around 3 to 6 days and is employed to decrease the quantity of vessels needed in a seed train. This aids in achieving higher-density reactor seeding and creating high-density seed banks.
Key benefits include its simplicity, reduced equipment requirements, and the minimal need for medium optimization. This method is particularly useful for quickly generating high cell densities without emphasizing productivity.
Operational considerations
Operationally, N-1 perfusion maintains a high medium exchange rate to ensure cells remain in their optimal growth phase. The short duration simplifies logistics and minimizes the need for extensive equipment.
Additionally, it provides higher seeding densities to production reactors, which is advantageous for downstream processes. However, since productivity is not a primary concern, it is less suitable for the final production stages.
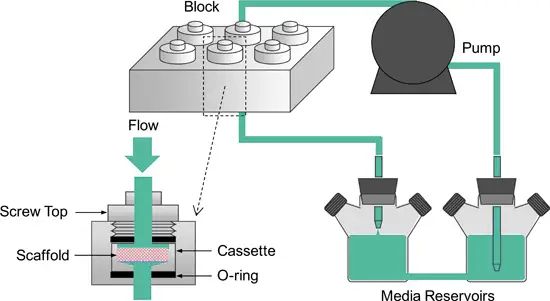
Concentrated fed-batch perfusion
Concentrated fed-batch perfusion uses alternating tangential flow filtration (ATF) or tangential flow filtration (TFF) to retain both cells and products within the bioreactor.
This method increases product amounts, making it great for stable products with low productivity in batch or fed-batch processes.
The concentrated environment enhances downstream processing efficiency by eliminating the need for additional concentration steps. Typically, this process runs for 14-20 days and targets high titers suitable for downstream applications.
Process requirements
Concentrated fed-batch perfusion is moderately complex, requiring ATF or TFF systems with filters that can retain the product within the vessel.
The medium exchange rate must be carefully controlled to achieve the desired product titers. This method works best for strong products that can handle intense conditions and has quality risks like fed-batch processes. Effective management of medium exchange is critical to maximizing titer and maintaining process stability.
Intensified fed-batch perfusion
Intensified fed-batch perfusion is like concentrated fed-batch, but with continuous product removal during the process.
This approach is advantageous for labile products and scenarios requiring high production concentration without additional downstream concentration steps. Shorter product retention times improve quality control and enable semicontinuous downstream purification. Run durations are generally 16-25 days, with medium exchange rates optimized for cost efficiency.
Comparison with concentrated fed-batch
Compared to concentrated fed-batch, intensified fed-batch offers higher total product generation and improved quality control due to continuous product removal.
Better suited for less stable products, as the reduced residence time lowers the risk of degradation. Additionally, it allows for the use of TFF or ATF filters to produce a clarified product stream ready for downstream processing. While both methods are moderately complex, intensified fed-batch tends to be more efficient and better for specific applications.
Continuous perfusion
Continuous perfusion aims to maintain steady-state conditions, ensuring consistent productivity and product quality over extended periods, typically ranging from 20 to 60 days.
This method uses a small amount of bleed to keep cells alive and at the right density, which helps improve efficiency. Optimized medium exchange rates ensure high efficiency and tight process control, making continuous perfusion ideal for long-term, stable production.
Long-term stability and efficiency
The primary advantage of continuous perfusion is its ability to sustain high product titers and quality with minimal variability. This method is particularly beneficial for integrating with continuous downstream bioprocessing.
Despite the longer time required for process optimization and the higher risk associated with extended operations, the benefits of superior quality control, reduced need for seed trains, and scalability make continuous perfusion a valuable option. Additionally, automation-friendly steady-state operations help mitigate the complexity, making it a viable choice for large-scale production.
Comparing perfusion techniques: Key differences and considerations
When it comes to optimizing bioprocesses, selecting the right type of perfusion is crucial.
Each perfusion technique, N-1 perfusion, concentrated fed-batch perfusion, intensified fed-batch perfusion, and continuous perfusion, offers unique advantages and operational considerations. Understanding these differences can help bioprocess engineers and project managers make informed decisions that enhance productivity, product quality, and efficiency.
The following table provides a comprehensive comparison of the four perfusion types, highlighting their primary goals, typical durations, medium exchange rates, complexity, cell retention methods, product removal strategies, suitability for labile products, quality control, operational considerations, scalability, risk of system fouling, and cost efficiency. This overview aims to guide you in selecting the most appropriate perfusion method for your specific bioprocess needs.
| Feature/Aspect | N-1 Perfusion | Concentrated Fed-Batch Perfusion | Intensified Batch Perfusion | Continuous Perfusion |
|---|---|---|---|---|
| Primary Goal | High cell densities | Increased product titer | Higher total product generation | Steady-state productivity |
| Typical Duration | 4-7 days | 14-20 days | 16-25 days | 30-90 days |
| Medium Exchange Rate | High, to maintain logarithmic growth | Moderate, to achieve target final titers | Optimized to minimize cost per gram of product | Optimized for efficiency and control |
| Complexity | Low | Moderate | Moderate | High |
| Cell Retention Method | Filtration or settling | ATF or TFF | ATF or TFF | Typically filtration |
| Product Removal | Not prioritized | Retained in bioreactor | Continuously removed | Continuously removed |
| Suitability for Labile Products | Less suitable | Suitable | Highly suitable | Highly suitable |
| Product Quality Control | Less emphasis | Similar to fed-batch | Improved due to continuous removal | Superior due to steady-state |
| Operational Considerations | Simplifies seed train operations | Requires stable product, moderate run length | Shorter retention improves quality, semicontinuous downstream | Long optimization, automation-friendly |
| Scalability | High | High | High | High |
| Risk of System Fouling | Low | Moderate | Moderate | Moderate to High |
| Cost Efficiency | High | Moderate | High | Moderate, balanced by fewer seed trains |
The impact of perfusion bioreactor on bioprocess efficiency
Perfusion bioreactor has revolutionized the field of bioprocessing by offering unparalleled efficiency, productivity, and product quality. This continuous method of culturing cells ensures a constant supply of fresh nutrients while removing waste, creating an optimal environment for cell growth and protein production.
Whether it's the rapid cell density increase in N-1 perfusion, the high product titers achieved with concentrated fed-batch, the enhanced control in intensified fed-batch, or the long-term stability of continuous perfusion, each technique provides unique benefits that cater to different stages and needs of bioprocessing.
By adopting perfusion bioreactor, bioprocess engineers can achieve higher yields, better consistency, and more efficient use of resources. As the biotechnology landscape evolves, mastering these perfusion techniques will be essential for staying ahead and driving forward innovations in biopharmaceutical production.
Frequently Asked Questions (FAQ)
Bioreactor perfusion is a continuous cell culture method that maintains optimal growth conditions by constantly supplying fresh nutrients and removing waste. This process enhances cell productivity and product quality by keeping the cells in an ideal environment.
Perfusion offers several benefits, including higher cell densities, increased product yields, improved product quality, and more efficient use of resources. It also allows for better control over the production process and can be scaled more easily to meet production demands.
Each perfusion type has unique features: N-1 perfusion focuses on rapidly increasing cell density, concentrated fed-batch perfusion aims for high product titers, intensified fed-batch perfusion enhances product generation and quality control, and continuous perfusion maintains long-term steady-state production with high consistency.
Factors to consider include the specific production goals, the stability of the product, the required cell density, the duration of the production run, and the complexity of the operation. Each perfusion method has different strengths that can be matched to the needs of the process.
Yes, bioreactor perfusion can be seamlessly integrated with downstream processes. Filtration methods used in perfusion provide clarified permeate that can be directly linked to downstream operations, improving overall process efficiency and product quality.