Redefine Optimisation with eBAG 3D Mixer
Smart liquid handling solutions in bags
Innovative and reliable single-use bag solutions for the biotech sector with eBAG 3D Mixer.
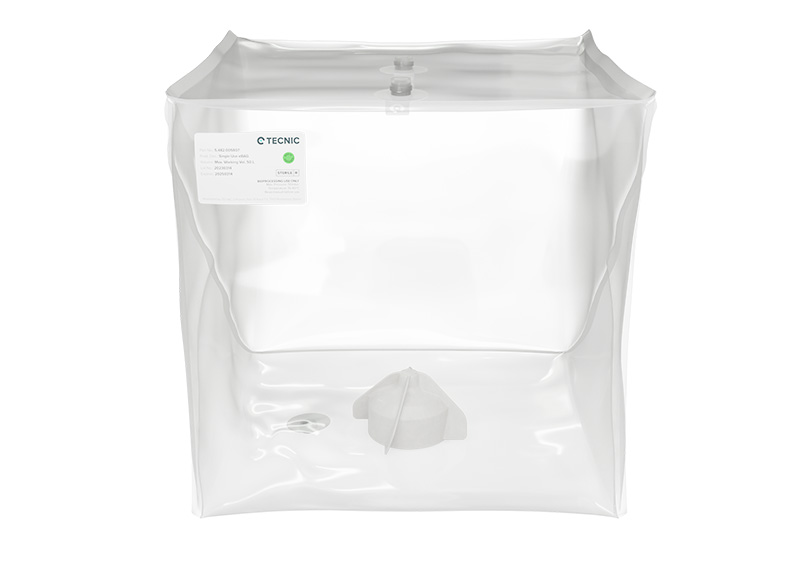
In the evolving landscape of Biotech, the single-use eBAG 3D Mixer optimize fluid dynamics for efficient mixing, save storage and easy transportation of liquids. Engineered with a unique 3D design, TECNIC eBAG 3D Mixer are reshaping the biotech sector by enhancing fluid dynamics for unparalleled quality results.
Produced under stringent aseptic conditions and subjected to comprehensive sterilization, these bags ensure high sterility and minimal contamination risk. With customizable features accommodating specific needs for size, configuration, and fittings, they seamlessly integrate into manufacturing processes.
Developed and Manufactured in the TECNIC ISO7 Clean Room, these 3D bags are constructed with high-quality materials and undergo gamma irradiation, making them ready-to-use according to the strictest pharmaceutical industry standards. Available in various sizes (50L, 100L, 200L, and 500L), these bags withstand rigorous handling, ensuring the safe containment of biopharmaceutical products. Meticulously designed to work seamlessly with TECNIC ePLUS® Mixer SU ⇀, they are manufactured to perfectly fit into the tank and adapt to the fully magnetic mixer for a secure and efficient process.
eBAG 3D Mixer for a superior solution
Film technology for enhanced bioprocess
Our TECNIC Film Technology is specifically engineered for high-quality. Every one of our Single-Use products distinguishes itself by its unique five-layer structure, with each layer serving a specific function to optimize the integrity, durability, longevity and the sterility of the equipment.
High standards in bioprocessing
Bag properties







