eBAG 3D STR for single use bioreactor solution
Dedicated to overcoming the limitations of single-use bag systems
Advanced eBAG 3D STR for dual-purpose single fermenter/bioreactors
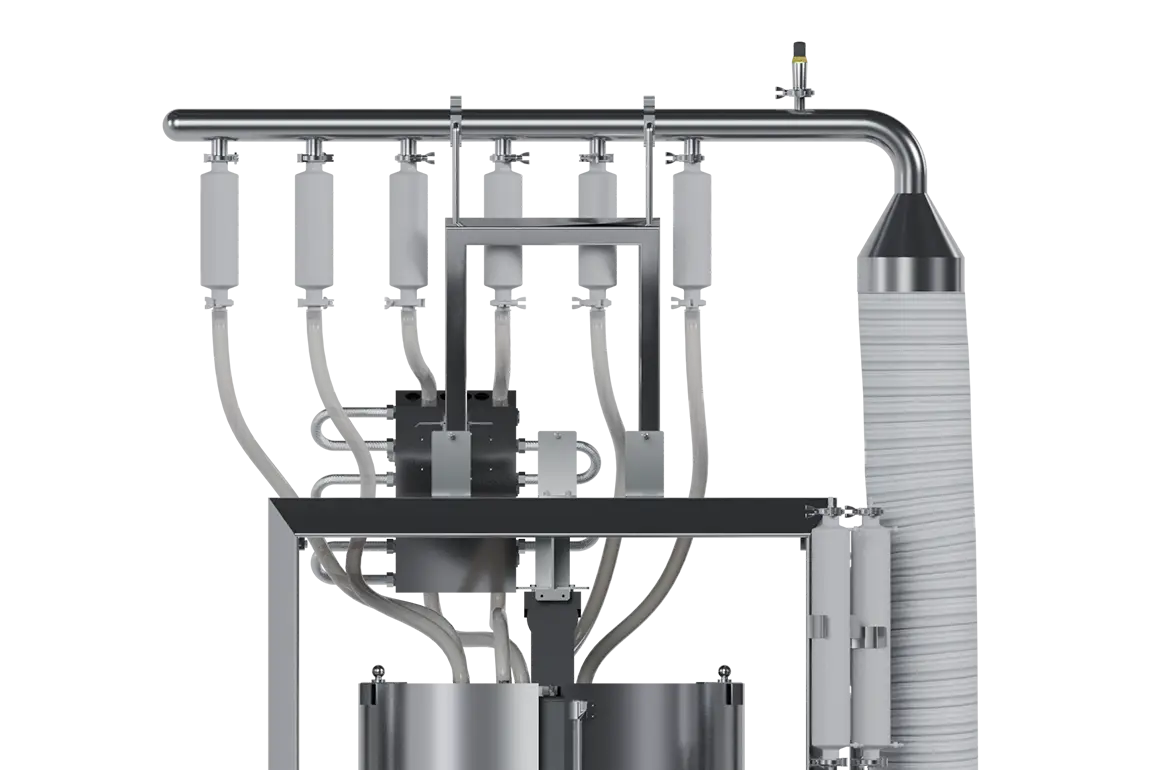
In the ever-evolving landscape of Biotech, TECNIC's Single-Use eBAG 3D STR is ingeniously designed to seamlessly integrate with our dual-purpose Single-Use bioreactor. Devoted to overcoming the inherent limitations in single-use systems, the eBAG 3D STR stands as a purpose-built, cutting-edge solution tailored for mammalian culture. As well as, this innovative system is meticulously crafted to tackle specific challenges, offering a transformative leap in the capabilities of microbial bioprocessing.
Produced under stringent aseptic conditions and subjected to comprehensive sterilization, these bags ensure high sterility and minimal contamination risk. With customizable features catering to specific size configurations and fittings, they seamlessly integrate into manufacturing processes.
Developed and Manufactured in TECNIC ISO7 Clean Room, eBAG 3D STR is built tough with high-quality materials and undergo gamma irradiation. As a result, they are ready-to-use according to the strictest pharmaceutical industry standards and are available in various sizes (30L up to 1000L). They can withstand rigorous handling to ensure the safe containment of biopharmaceutical products. Perfectly designed to work with TECNIC ePILOT and ePROD Single Use Bioreactor ⇀, manufactured to perfectly fit into the tank and fully adapt with the fully magnetic mixer to provide assurance in your process.
Guaranteed sterility for sensitive bioprocesses
Film technology for enhanced bioprocess
Our TECNIC Film Technology is specifically engineered for high-quality. Every one of our Single-Use products distinguishes itself by its unique five-layer structure, with each layer serving a specific function to optimize the integrity, durability, longevity and the sterility of the equipment.
High standards in bioprocessing
Bag properties

Our sales team is ready to assist you with the most technical details.
Connect with our team today. Whether you have specific questions or are just beginning to explore, we're here to guide and assist. Reach out now and let's shape the future together.