The selection of the right bioreactor is crucial to ensure success in bioprocesses, whether in the laboratory setting or in industrial-scale production. There are several types of bioreactors, and choosing the right one involves considering several technical and economic factors, which directly affect efficiency, productivity and operating costs. Here is a guide to help you make the best choice.
Type of bioreactors
The first thing to consider is the type of biotechnological process you are going to carry out. Bioprocesses generally fall into three main categories, and for each there are three types of bioreactors that are most suitable:
- Batch: In this type of process, all elements are added at the start and the culture is left to ferment for a defined time. It is simpler and easier to control, making it ideal for small-scale experiments or processes with stable products.
- Fed-batch: Here nutrients are added during the fermentation process to prolong the growth phase. It is suitable for higher volume and more complex productions, as it allows more control over cell growth and metabolite production.
- Perfusion: In this method, nutrients are continuously added and products are removed simultaneously. It is the best option for large-scale processes where product consistency is key, but also requires a higher level of automation and monitoring.
Type of cell or microorganism
Another determining factor is the type of cell or microorganism you are working with. Each cell type has specific oxygen, temperature, pH and nutrient requirements that affect the choice of bioreactor. Therefore, knowing the types of bioreactors available and how they are adapted to each cell is essential.
- Animal cells: Require bioreactors with gentle agitation to avoid damage, advanced aeration systems to maintain adequate oxygen levels, and precise control of pH and temperature.
- Bacterial cells: May require a bioreactor with more intense agitation for homogeneous mixing and more vigorous aeration to meet oxygen needs.
- Yeasts or fungi: They tend to grow in more viscous media and therefore require a bioreactor with a more robust agitation system.
Scalability
It is important to consider the potential for scalability from the start. Bioreactors used in initial research or development should be easy to scale up for higher volume production.
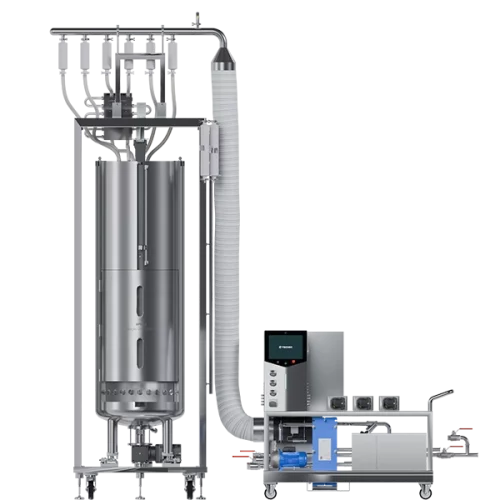
At TECNIC, we offer bioreactors designed for a smooth transition from laboratory to full-scale production. Our equipment is designed to maintain consistency and reproducibility at every stage of scale-up, optimising both process quality and efficiency. If you start with a small laboratory bioreactor (1-10 L), our systems are ready to scale up to 4000 L volumes, without compromising control over critical parameters such as oxygenation or agitation. At each stage, there are different types of bioreactors to suit the needs of the process.
Control and automation requirements
The level of control and automation required will depend on the type of process and the consistency you want to achieve. Modern bioreactors typically include automated pH, oxygen, temperature and nutrient control systems. Investing in a more advanced monitoring and control system can offer great benefits, especially in complex or large-scale bioprocesses.
Automation can reduce human error, improve reproducibility and facilitate real-time process optimisation. If your bioprocess requires strict accuracy and consistency, a system with a higher degree of automation will be essential.
Single-use vs reusable bioreactors
Finally, a key consideration is whether to use reusable or single-use bioreactors.
- Single-use bioreactors: These are ideal for small-scale processes or batch production. They eliminate the need for cleaning and sterilisation between cycles, which saves time and reduces the risk of contamination. They are also flexible and can be quickly changed for different projects.
- Reusable bioreactors: These are more suitable for large-scale processes, where continuous production is necessary. Although they require more time and resources for cleaning and validation, their prolonged use can be more cost-effective in the long term.
Conclusion
Choosing the right bioreactor depends on a number of factors, from the type of bioprocess to the specific requirements of the cells or microorganisms, scale-up, and automation and control needs. Carefully evaluating these aspects will allow you to select the bioreactor that best suits your needs, thus optimising the efficiency and productivity of your biotechnological processes.
At TECNIC, we offer a wide range of bioreactor types for all stages of bioprocessing, from laboratory to industrial production. Our expertise and flexible solutions allow you to scale up efficiently, while optimising both time and resources. Contact us for more information on how we can help you find the perfect bioreactor for your project.
Frequently Asked Questions (FAQ)
The most common types of bioreactors are batch, fed-batch and perfusion. Each has specific applications depending on the biotechnological process and project requirements.
The choice of bioreactor will depend on several factors such as the type of cell or microorganism, the production volume and whether you need to scale up the process. For research laboratories, batch bioreactors tend to be a popular choice, while perfusion bioreactors are preferred for larger scales and continuous processes.
Reusable bioreactors are designed for long-term processes and require cleaning and sterilisation between uses, while single-use bioreactors eliminate the need for these processes, making them ideal for projects that require flexibility and reduced risk of contamination.
When selecting a bioreactor, it is important to consider cell type, production volume, ease of scale-up and automation and control needs. In addition, economic factors, such as operating cost and set-up time, also play a crucial role.
Scale-up is recommended when laboratory tests are consistent and production requires higher volumes or efficiency.